Services
CoreX Regulatory Labelling Service:
A “one-stop-shopping”
The CoreX team offers a comprehensive labelling package that goes beyond the provision of the required translations.
The service is both:
- advisory / strategic – providing regulatory guidance and labelling expertise
- operational – providing experienced resources and process know-how.
The service covers all EU Procedures (New MAA, variations, renewals, line extensions etc.), including:
- Support to set up / optimise the English text
- Optimisation of the language versions
- Guiding the translators e.g. by commenting on critical text elements and providing reference materials
- Including a proofreading step by a second native speaker
- Applying appropriate in-house QC steps, reducing / eliminating possible QC steps for the client
- Management of the whole process between Day 0-235 (+25)
- Complete coordination of all activities related to the set-up of the language versions
- Management of a review by client affiliates (on request)
- All authority interactions related to the linguistic review process, including submissions
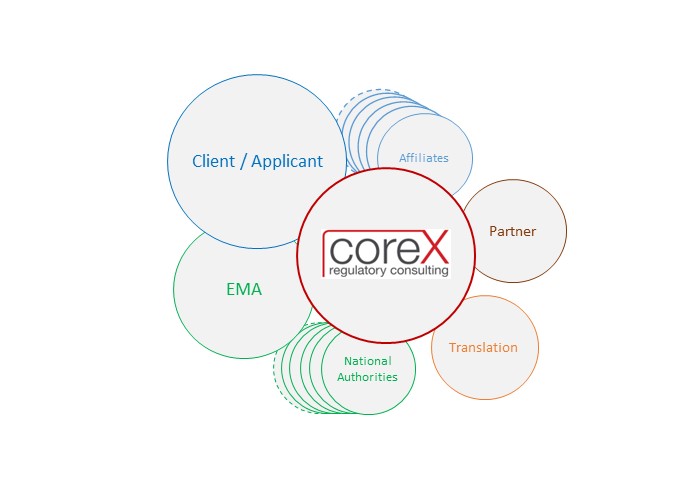
Minimise involvement of client resources, maximise quality
Interface function of CoreX
Read more about our “one-stop-shopping” service in our flyer
CoreX Produkt Information Support [PDF 1,8 MB]





